- HOYA data on file. CTM-23-029, HOYA Medical Singapore, Pte. Ltd, 2023.
- Ribeiro et al. Analysis of Daily Visual Habits in a Presbyopic Population. J Ophthalmol. 2023 Apr 8;2023:6440954.
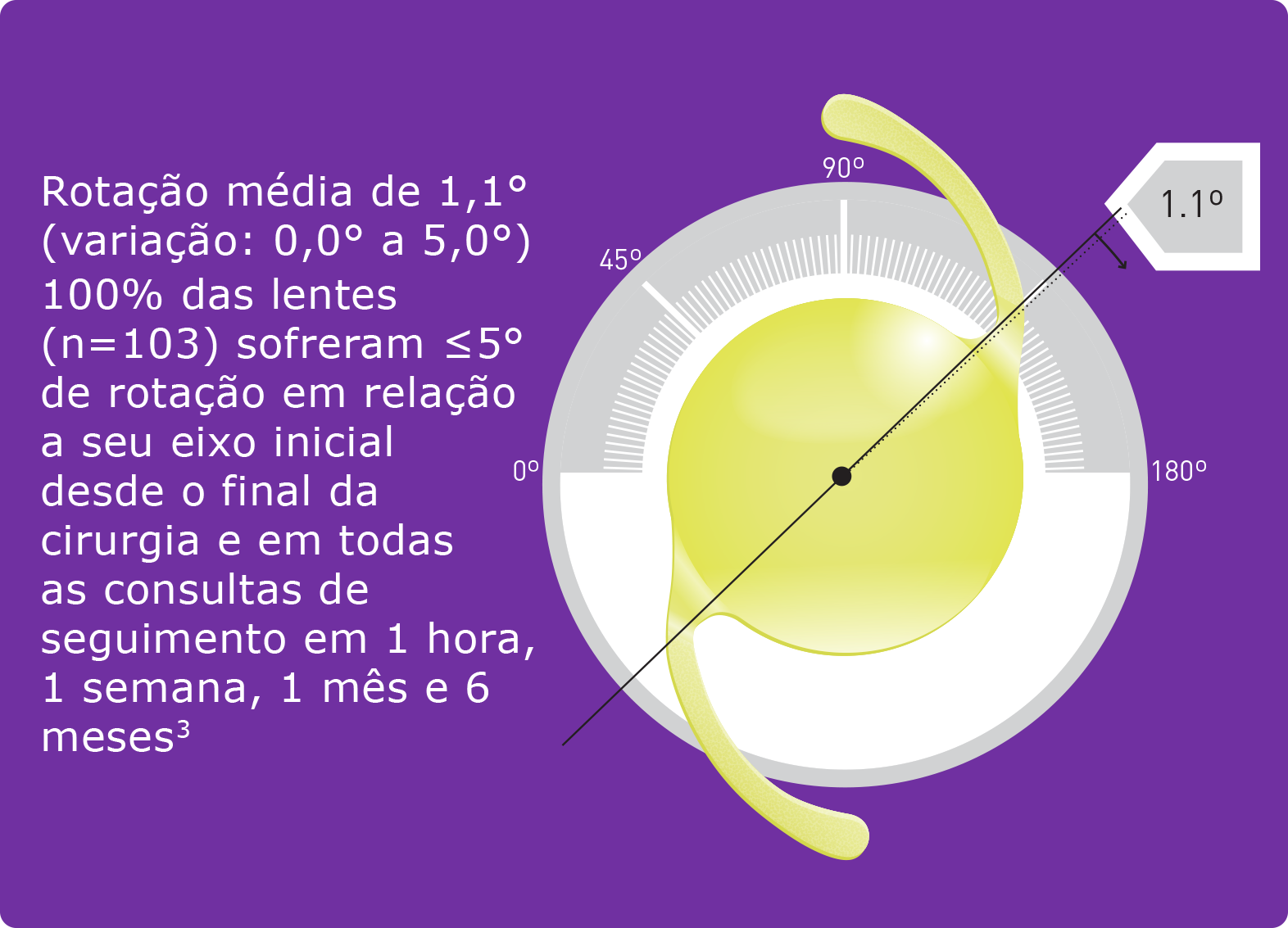
- Schartmueller, D. et al. (2019): True rotational stability of a single-piece hydrophobic intraocular lens. In: The British journal of ophthalmology 103 (2), p. 186–190.
- Pérez-Merino, P.; Marcos, S. (2018): Effect of intraocular lens decentration on image quality tested in a custom model eye. In: Journal of cataract and refractive surgery 44 (7), p. 889–896.
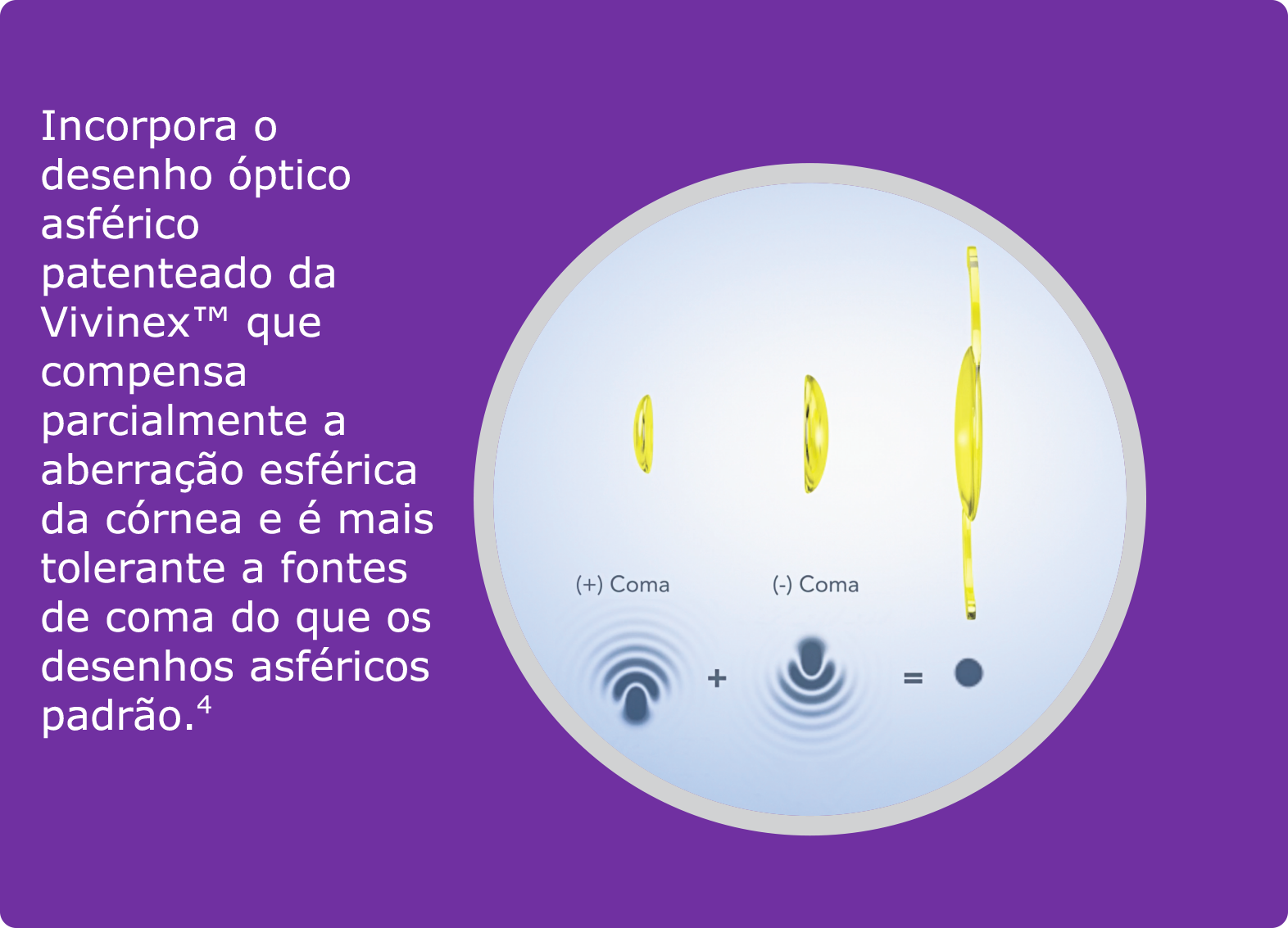
- Tandogan, T. et al. (2021): In-vitro glistening formation in six different foldable hydrophobic intraocular lenses. In BMC Ophthalmol 21, 126.
- Miyata, A. et al. (2001): Clinical and experimental observation of glistening in acrylic intraocular lenses. In: Japanese journal of ophthalmology 45 (6), p. 564–569.
- Auffarth et al. (2023) Randomized multicenter trial to assess posterior capsule opacification and glistenings in two hydrophobic acrylic intraocular lenses. Sci Rep 13, 2822.
- Leydolt, C. et al. (2020): Posterior capsule opacification with two hydrophobic acrylic intraocular lenses: 3-year results of a randomized trial. In: American journal of ophthalmology 217 (9), p. 224-231.
- Giacinto, C. et al. (2019): Surface properties of commercially available hydrophobic acrylic intraocular lenses: Comparative study. In: Journal of cataract and refractive surgery 45 (9), p. 1330–1334.
- Werner, L. et al. (2019): Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. In: Journal of cataract and refractive surgery 45 (10), p. 1490–1497.
- Matsushima, H. et al. (2006): Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. In: Journal of cataract and refractive surgery 32 (6), p. 1035–1040.
- Farukhi, A. et al. (2015): Evaluation of uveal and capsule biocompatibility of a single-piece hydrophobic acrylic intraocular lens with ultraviolet-ozone treatment on the posterior surface. In: Journal of cataract and refractive surgery 41 (5), p. 1081–1087.
- Eldred, J. et al. (2019): An In Vitro Human Lens Capsular Bag Model Adopting a Graded Culture Regime to Assess Putative Impact of IOLs on PCO Formation. In: Investigative ophthalmology & visual science 60 (1), p. 113–122. 45 (6), p. 847–853.
- Nanavaty, M. et al. (2019): Edge profile of commercially available square-edged intraocular lenses: Part 2. In: Journal of cataract and refractive surgery Our trifocal family of IOLs designed to advance patients' vision.
Disclaimer Information contained is intended for health care professionals. Please refer to the Instructions For Use for the intended purpose and a full list of indications and contraindications. Some of the products and/or specific features as well as the procedures featured in this document may not be VivinexTM GemetricTM Plus – Is designed to provide excellent near vision and well balanced distance and intermediate vision.1 approved in your country and thus may not be available there. Design and specifications are subject to change without prior notice as a result of ongoing technical development. Please contact our regional representative regarding individual availability in your country. HOYA, Vivinex, Gemetric, and multiSert are trademarks of the HOYA Corporation or its affiliates.©2023 HOYA Medical Singapore Pte. Ltd. All rights reserved.
| Vivinex ™ Gemetric™ / Vivinex ™ Gemetric™ Plus | Vivinex ™ Gemetric™ Toric / Vivinex ™ Gemetric™ Toric Plus | |
| Nome do modelo | XY1-G / XY1-GP | XY1-GT / XY1-GPT |
| Injetor | multiSertTM preloaded | multiSertTM preloaded |
| Material | Vivinex Hidrofóbico | Vivinex Hidrofóbico |
| Design | Peça única Asférica C-loop | Peça única Asférica C-loop |
| Zona Óptica | 6.00 mm | 6.00 mm |
| Tamanho Total | 13.00 mm | 13.00 mm |
| Filtro UV e 430 nm | Sim | Sim |
| Angulação de Alça | 0° | 0° |
| Dioptrias | +10.0D a +30.0D incremento de 0.5D | +10.0D a +30.0D incremento de 0.5D |
| Adição de Dioptrias | +1.75D para Intermediária / +3.50D para Perto | +1.75D para Intermediária / +3.50D para Perto |
| Ponto Focal | 37.5cm para Perto / 75.0cm para Intermediária | 37.5cm para Perto / 75.0cm para Intermediária |
| Cilindros e Dioptrias no plano da lente | T2=1.00D T3= 1.50D T4= 2.25D T5= 3.00D T6= 3.75D |
|
| Cilindros e Dioptrias no plano da córnea | T2= 0.69D T3= 1.04D T4= 1.56D T5= 2.08D T6= 2.60D |
|
| Qtd. Anéis Difrativos | 08 Anéis | 08 Anéis |
| Constante A Nominal | 119.0 | 119.0 |
| SRK T | 118.998 | 118.998 |
| SRK II | 118.998 | 118.998 |
| Barret | 1.88 | 1.88 |
| Haigis | A0 -0.0199 A1 0.3437 A2 0.1620 |
A0 -0.0199 A1 0.3437 A2 0.1620 |
| Hill-RBF | 119.1 | 119.1 |
| HofferQ | 1.789 | 1.789 |
| Holladay - 1 sf | 1.789 | 1.789 |
| Incisão | 2.2 mm | 118.998 |
| Anvisa | 80686360342 | 80686360341 |
* A constante-A é apresentada como um ponto de início para o cálculo do grau das lentes. Ao calcular o grau das lentes, recomendamos que os cálculos sejam feitos de forma individual, com base nos equipamentos usados e na própria experiência do cirurgião
** Essas constantes otimizadas de cálculo de potência da lente intraocular, publicadas pela IOLCon em seu site https://iolcon.org, foram calculadas a partir de 2.857 resulta ponto de início para otimizações constantes individuais. As informações disponíveis no site são baseadas em dados obtidos de outros usuários e não da HOYA Surgical Optics (“HSO”). A HSO, portanto, não assume qualquer responsabilidade pela exatidão, integridade e atualidade do conteúdo do site mencionado.
Por favor faça download para visualizar as Especificações

Apresentando Vivinex™ Gemetric™
Nossa família trifocal de LIOs destinada a proporcionar amplitude total de visão
Vivinex™ Gemetric™ — Destinada a proporcionar excelente visão de longe e visão intermediária e de perto bem balanceada.1
Vivinex™ Gemetric™ Plus – Destinada a proporcionar excelente visão de perto e visão de perto e intermediária bem balanceada.1

O pareamento proporciona uma grande amplitude de visão continuamente
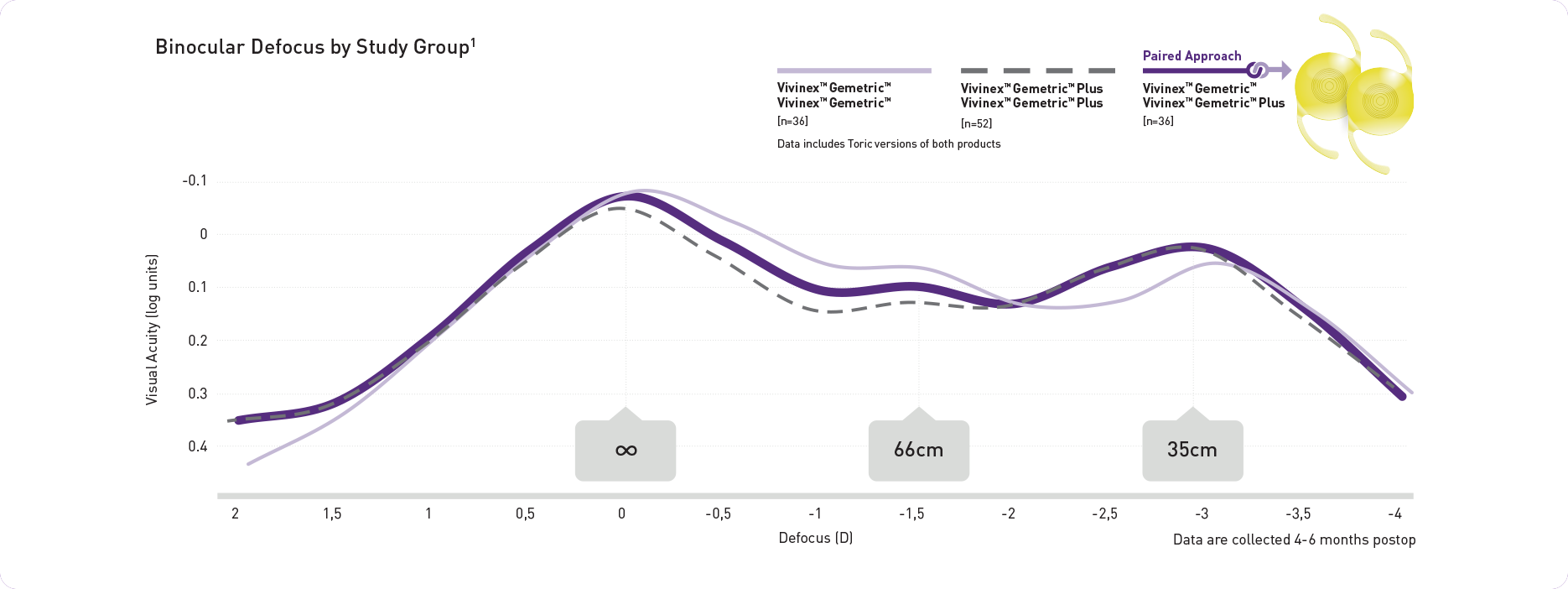
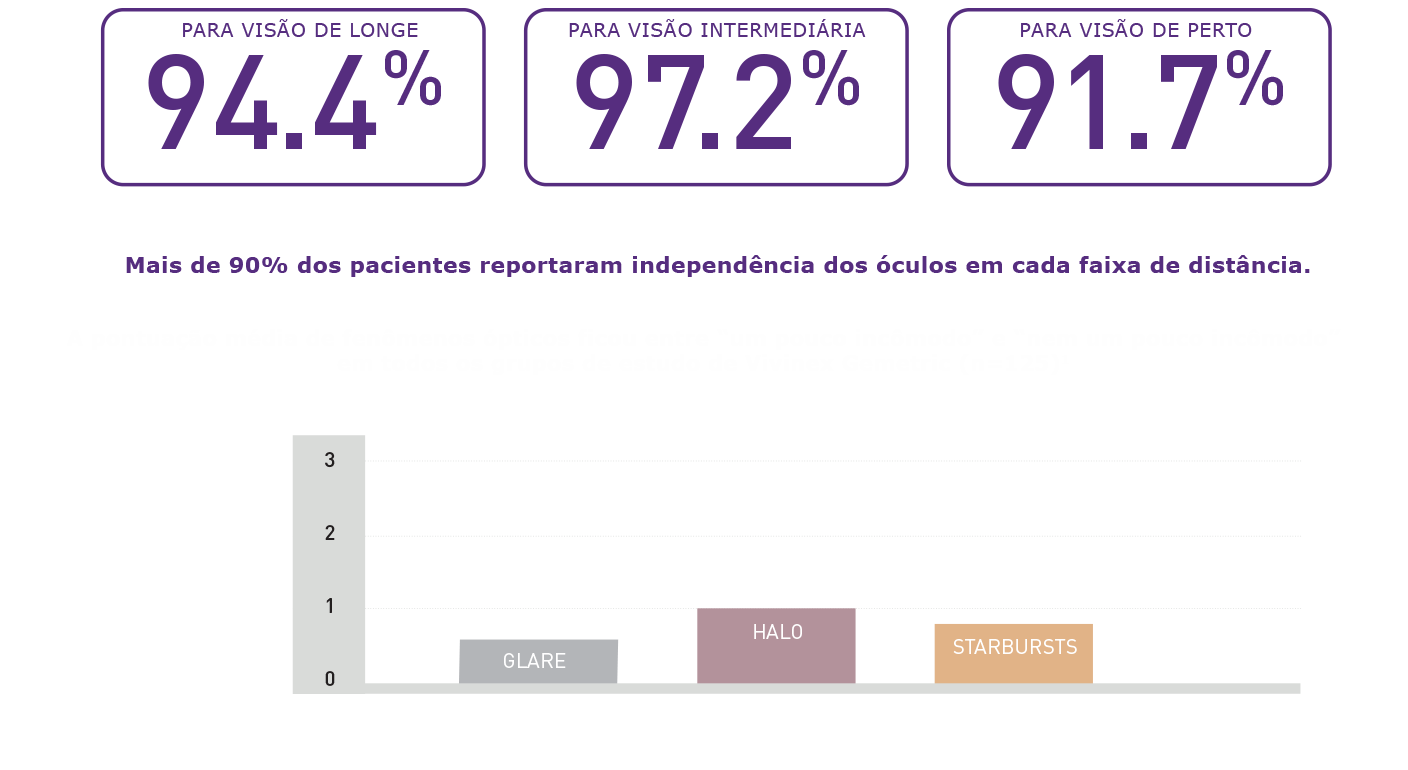
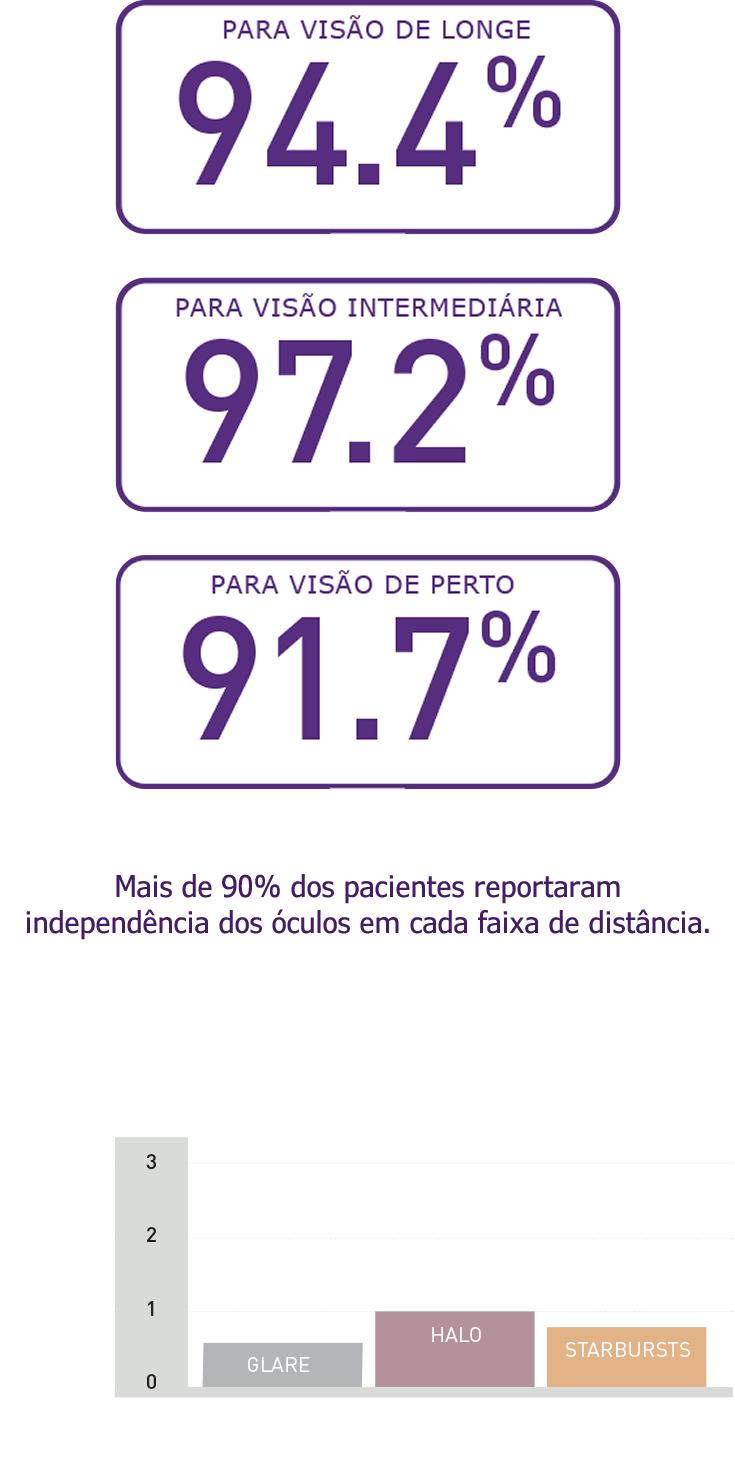
Resultados Reportados pelos Pacientes
Alta taxa de independência dos óculos relatada pelos próprios pacientes e de satisfação com o pareamento de Vivinex™ Gemetric™ e Vivinex™ Gemetric™ Plus e meses após a cirurgia (n=36).1

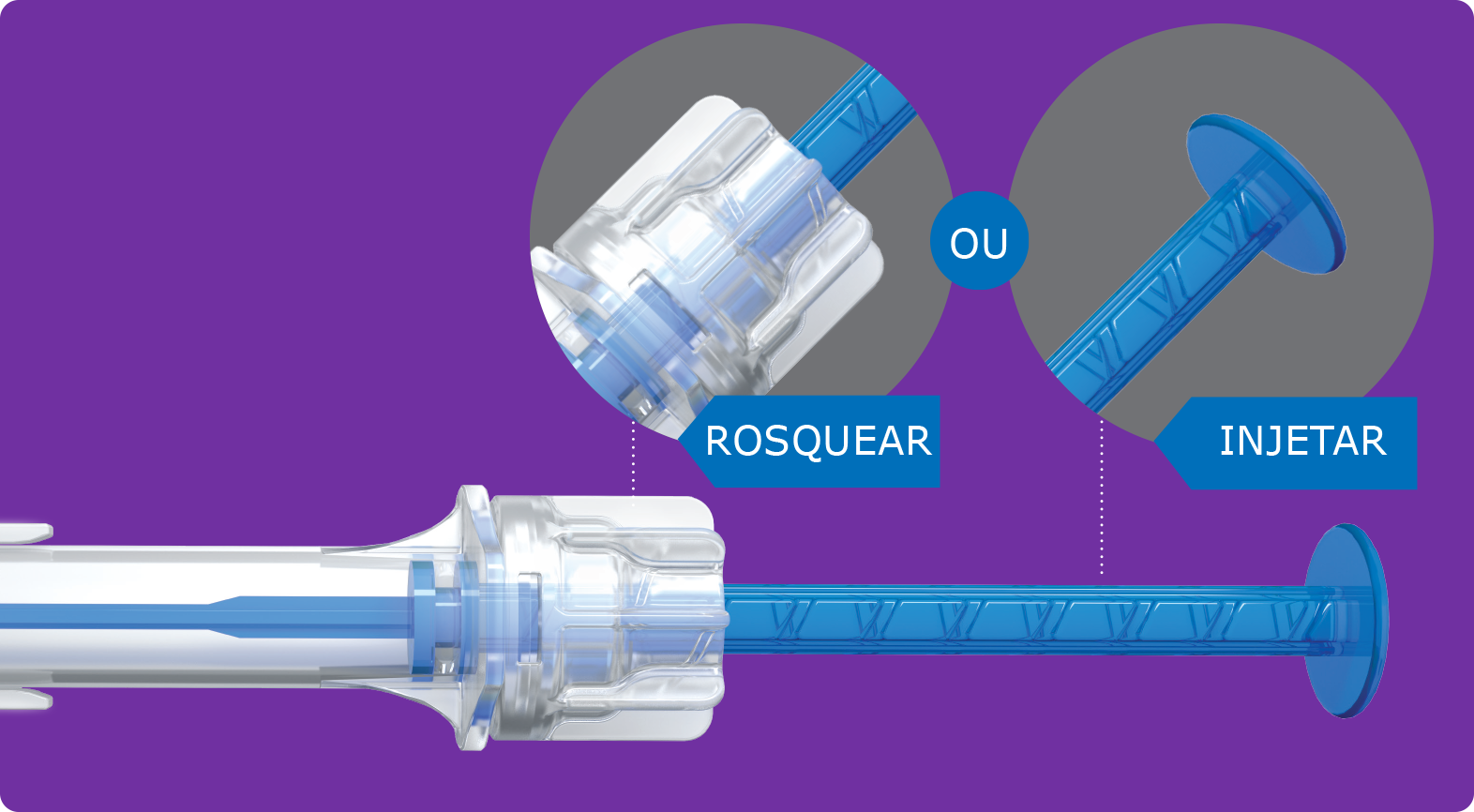
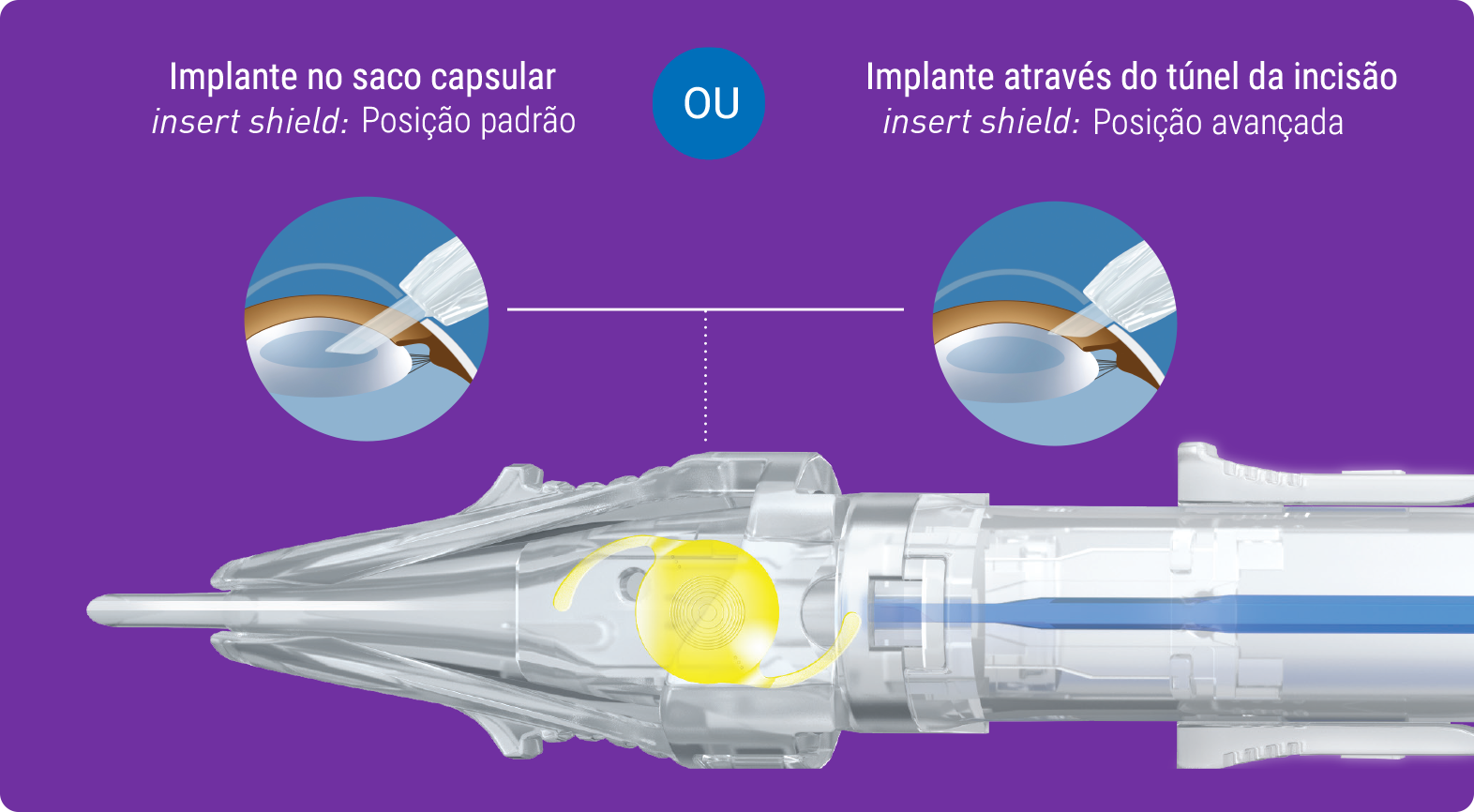
Implantada através de nosso injetor pré-carregado multisert™